There are many ways that Compass can become a part of your team and produce your desired outcome; either for just one aspect of your program, for the entire development project, or anything in between. Once a concept has been vetted through basic research, and deemed viable for pre-clinical evaluation and human clinical trials (requiring the establishment of GLP and GMP systems), the simplified product development workflow looks like this:
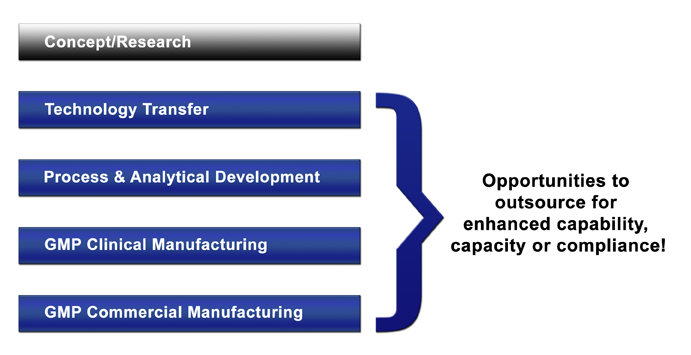
Depending on your internal capabilities, you may need to outsource any or all of these tasks to an external contractor(s) who has the necessary resources from both a facility and personnel perspective. Compass can become an integral part of your team, giving you the necessary additional bandwidth by acting on your behalf as if we were one of your own employees, partnering with you at any given step to facilitate getting the work done. Since we know the appropriate companies in the CMO business, and we understand how to do it in compliant and cost efficient manner, our support to you can occur with any or all of these 4 simple steps:
- Scope: The initial conversations with you will occur at no cost. After establishing a CDA, we will discuss in detail where you are in the product development workflow, determine what work has been done to date, and then draft a strategy and scope of work (SOW) for the project, based on your corporate goals and objectives. The SOW will also include rough estimates of costs, based on our previous experience.
- RFP: Once the scope has been determined and you have made the decision to proceed to the next step, we will act as your Technical Operations person and draft a Request for Proposal (RFP), which will be sent to a number of appropriate CMOs with capability consistent with your needs. The RFP will be reasonably detailed, giving the contractor enough information to provide a proposal with accurate cost estimates and timing. All replies will then be summarized from a cost/benefit perspective and a presentation made to you for final selection. Depending on the scope of the project and our personal experience with a given CMO, it may be valuable to do an audit of their facility prior to contracting.
- Contracting: Once a decision has been made to move forward with a given CMO, detailed contracts will be drafted and negotiated, containing the terms and conditions of the agreed upon SOW. Contracts will be reviewed by legal personnel (yours and/or ours) as well, prior to approval, as these tend to be complex business documents.
- Execution: A project team will be assembled between Compass and the CMO, including personnel from your company as requested. This team will track and monitor the work throughout the duration of the project, with membership varying over time as necessary. Regular site visits to the CMO are many times a part of this monitoring process in order to maintain accountability and maximize performance; we will be your person in the plant. Regular communication will be maintained with you, with issues escalated as necessary for input and resolution. A final summary will document all relevant aspects of the project.
So, as you can see, we can deliver expertise with regard to a variety of business and technical related activities, providing only as much assistance as you need for a given phase of the product development workflow. We can support you with either a contract or time based agreement, which will be delineated and agreed upon prior to initiating a given step.


